Newfoundland & Labrador Prospectors Association (NLPA)
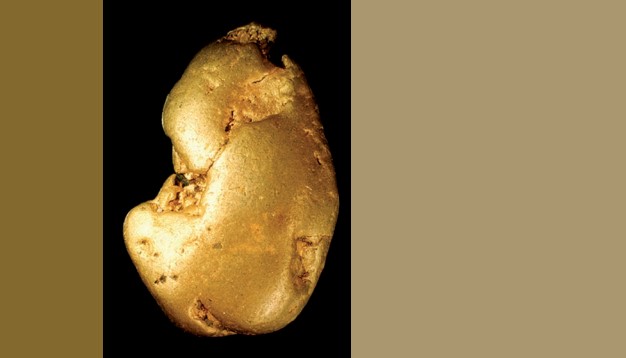
Gold prospecting by pan
Since the 1990s, prospecting for gold and oil — or «mineral prospecting» — has become one of the most active economic areas. This frenetic activity is directly related to increasing demand from emerging market economies, like China, Brazil and, more traditionally, India, and that of Western economies, which consider gold a valuerefuge. This has led, given its rarity of less than 4 milligrams per tonne of rock, to extreme pressure on the planet Earth, affecting its sustainability. In short, the richer its inhabitants the greater the demand.
The search process and subsequent extraction is known as «mineral exploration», defined as «the part of Geology which is interested in the search for minerals, rocks and energy resources with economic potential». Within mineral exploration, the most historical, traditional and cheap method would be alluvial prospecting or «prospecting by pan». This method and the associated technique — «panning» — is directly concerned with finding and assessing heavy metal deposits that indicate the existence of placer or residual deposits, and thus indirectly with determining the location of mineral and, therefore, geochemical anomalies. This requires the sampling of rivers and streams, which can lead to source or primary deposits of all types of minerals or also show up other companion minerals known as indicators or pathfinders.
This technique, logically used in the early stages of exploration, is not only employed for gold but also for substances known in their raw form as heavy minerals of commercial interest, such as platinoids and gems in the broad sense, including diamonds and technological and strategic minerals like titanium, zirconium, hafnium, rare soils, tin, tungsten and niobium-tantalum, the latter mined as «coltan».
A pan is a simple, cheap, easily obtained and easy-to-use recipient shaped like a dish, pan, bowl or basin, without sensors or electronics, which in the 21st century remains the most effective and efficient tool in mineral exploration. Some of its forms, as can be seen in this book and the accompanying video, are the result of empirical knowledge, materials close to hand, and the intuitive application of fluid mechanics, which is to say fluvial dynamics. Gold pans are used by millions of people worldwide for subsistence artisanal or small-scale mining, and are typically used in the Western world in the pursuit of panning as a hobby or sport.
CHARACTERISTICS OF GOLD
Gold, chemical symbol Au, occupies position 79 on the periodic table, between platinum and mercury, and belongs to Group 11 together with copper and silver. It is a relatively soft metal, ductile and malleable, and a good conductor of heat and electricity. Both gold and the other two elements in Group 11 show little similarity to the alkaline metals of Group 1.
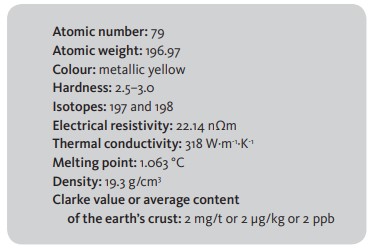
Gold is a fairly widespread chemical element in the earth’s crust but at very low concentrations, of around 4 mg/t (0.004 grams per tonne of rock). Comparing this to another element such as copper, the average content of the latter is 23,000 times that of gold at around 47 grams in each tonne of rock. This proportion is not consistent with their monetary values, as gold is only worth 3,200 times more than copper.
Another characteristic of gold is its high density relative to water. A litre of water weighs one kilogram, while the same volume of gold would weigh 19 kilograms and 300 grams.
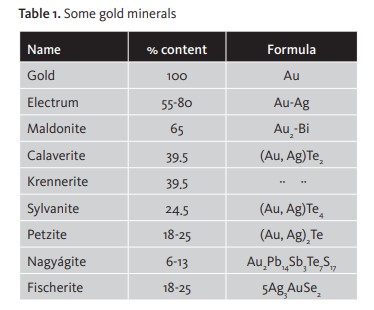
In the natural environment gold occurs as a native metal or alloyed with other metals, principally silver (Ag), copper (Cu), antimony (Sb), bismuth and metals of the platinum group. When the Ag content is higher than 20% it is called electrum. [Table 1]
One of the peculiarities of gold, due in part to its widespread diffusion in the earth’s crust compared to other metals of economic interest, is the large number of types of mineral deposits it can form, as either an element or mineral base, even as a by-product of other mineral ores.
GOLD IN NATURE
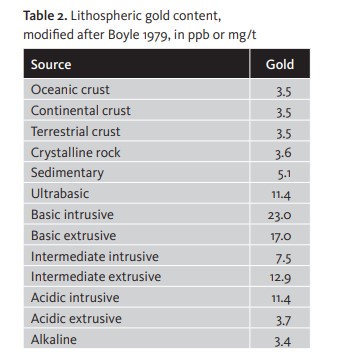
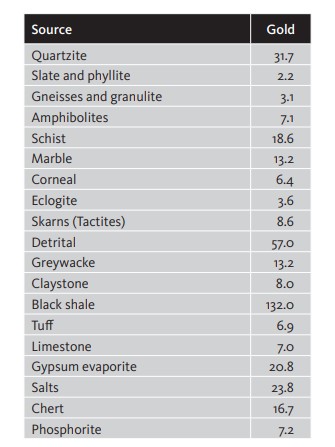
Most authors agree that the abundance of gold in Nature is equivalent to an average of 4 milligrams per tonne of earth, with highest levels of content occurring in basic and ultrabasic rock. [Table 2]
Among sediments the most significant are the modern detrital formations, sometimes called «placers», as well as the ancient rand conglomerates in South Africa and black shale.
Gold in water
Au is widely spread in groundwater and surface water, natural springs, sea water and so on, with an average fresh water content of 0.03 µg/l (although many authors believe it to be significantly lower) and 0.012 µg/l in marine waters; around 0.53 µg/l Au in geothermal systems.
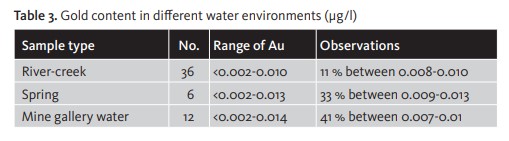
There are marked variations above and below this average, if we look at contents in specific types of water body, associated with different geological terrain. Camacho (1988) observes contents ranging from 0.02-0.06 ppb in unfiltered water from the gold district of Penedono (Portugal) and 0.002-0.01 ppb in filtered water from the same district. [Table 3].
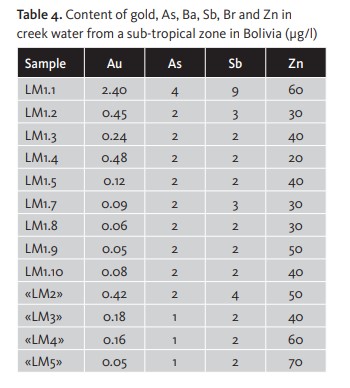
Another example performed by the same author in a creek near the gold and antimony mine at Los Machos (Bolivia) showed that at a single point, where ten samples (LM1.1-LM1.10) were taken at a sampling rate of 1 litre of water every 20 seconds (stream flow 3 m3/s), the gold values of different unfiltered samples are completely erratic, ranging from 0.05-2.4 mg/l. The high values of Au, arsenic (As), zinc (Zn) and antimony (Sb) observed in the first of the ten samples suggest the presence of gold particles. [Table 4]
Gold in vegetation
Numerous works from the mid-20th century tell us of the gold content in plants and animals.
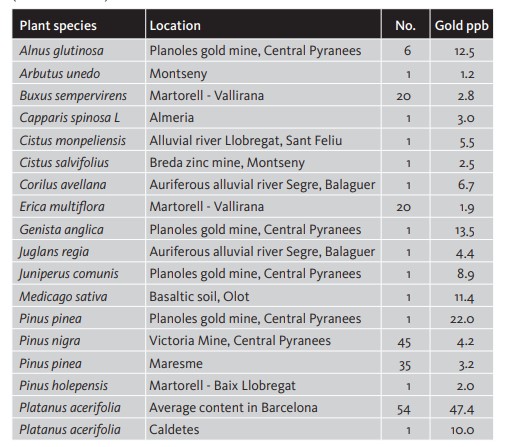
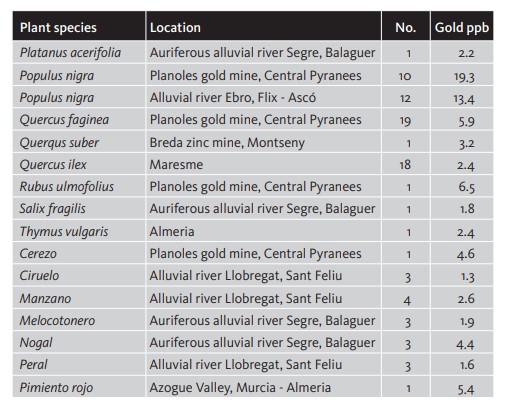
Gold concentrations in humus, matter that is halfway between soil and vegetation, are more evenly spread than in alluvial, colluvial and glacial deposits. The causes of gold presence in humus can be related to the gold retention capacity of organic matter, as well as a reduction effect or gold absorption by plants, mainly cyanogenic plants that, in order to dissolve vital elements such as Zn, produce cyanides that also dissolve gold (Shacklette 1970). Table 5. Gold concentration in some plant species (mg/t) of the Iberian Peninsula (Viladevall 2008).
Source: http://www.publicacions.ub.edu/refs/indices/08397.pdf
