Newfoundland & Labrador Prospectors Association (NLPA)
Lithium
Use of lithium has migrated from the classical use in glass and ceramics to batteries for portable electronics and electric cars. This has led to a renewed interest in lithium as a target element for exploration.
Lithium, at the top of the periodic table, was of little interest until the electronic revolution. With an atomic number of 3 and a weight of 6.9, lithium is exceptionally small and light, with high charge/radius ratio. It is now regarded as an exciting market commodity, with high specific heat capacity and high electrochemical potential, making lithium ideal in heat transfer technology where it is used in welding and metallurgical applications. High electrochemical potential and light weight make it amenable to battery applications. Lithium batteries have three times the energy of nickel hydride at one-third of the weight, and they operate at very low temperatures with a longer battery life.
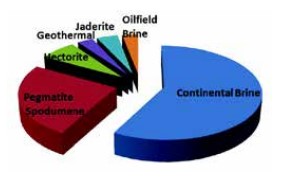
Lithium occurs in continental brines, geothermal or oilfield brines, and in pegmatite or hectorite-rich clay deposits. Continental brine accumulations host Li within the remnant brine itself, and within clay minerals interleaved with evaporite deposits. These deposits contain salts, carbonates, phosphates, borates, sulfate and clay minerals. The Chabyer salt lake in the Tibetan region is host to one of the largest known concentrations of Li with high grades of 0.12% Li occurring as the carbonate mineral Zabuyelite. Potash operations often produce by-product Li.
Pegmatite deposits host Li in spodumene, lepidolite (mica), with Li also substituting for Mg2+ in tourmaline and alumino-silicate minerals. Within pegmatites an early deposition of Quartzfeldspar may be followed by later deposition of spodumene or petalite, possibly with some contribution from late stage gaseous phases. Montmorillonite and other clays accumulate the small highly charged element.
Lithium Background Concentrations:
• Natural waters: 1-3 ppb
• Sea water: 170 ppb
• Hydrothermal waters: 30 ppm
• Soil samples: 20-200 ppm depending upon underlying host rock.
• Ultramafic rocks: 0.5 ppm
• Granitic rocks: 40-100 ppm
• Micaceous schists: 50-200 ppm
• Pegmatities: 100-2,000 ppm
• Continental brines: 50 ppb-2,000 ppm
» Great Salt Lake: 60 ppm Li
» Salton Sea: 200 ppm Li
» Chabyer salt lake: 0.12% Li
» Salar de Atacama: 200 ppm-0.18% Li
Exploration for lithium may be aided by the pathfinder geochemistry of K, Mg, B, F, Na, Ca, Li/K and Li/Mg ratios may be of interest. Brine water samples may also be analyzed for general chemistry including pH conductivity, alkalinity, total dissolved solids, and Cl or Br. Vegetation samples show anomalous Li in response to elevated groundwater, and indicator species may provide a useful exploration medium (Cannon, 1975). Enriched brine occurrences require three factors for economic accumulation: an enriched Li host source rock, a transport mechanism, and concentration, trapping or sorption mechanism. Exploration c onsiderations should include Li-enriched sources such as differentiated rhyolitic or granitic terrane, a groundwater or gaseous mechanism for Li transport and accumulation, and concentration or trapping via evaporation or clay sorption
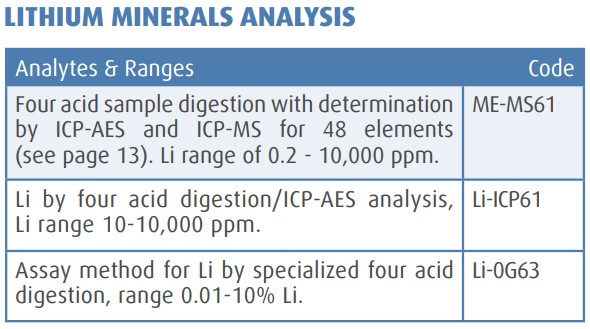
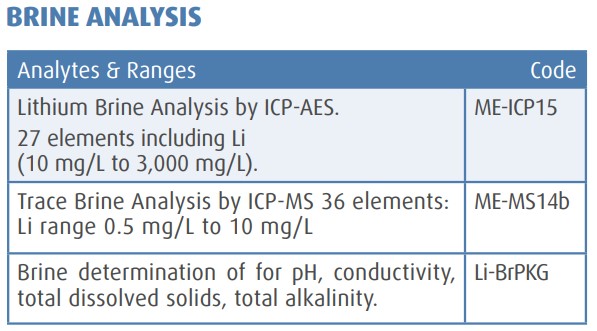

Source: https://www.nlprospectors.org/Mission/Education/Lithium_Technical_Note_2012.pdf
